Unveiling the Mystery: Understanding White and Red Reactions
Have you ever wondered why some chemical reactions produce a vibrant red color while others remain stark white? The answer lies in the fascinating world of chemical reactions and the intricate interplay of chemical compounds, electrons, and energy.
What is a Red Reaction?
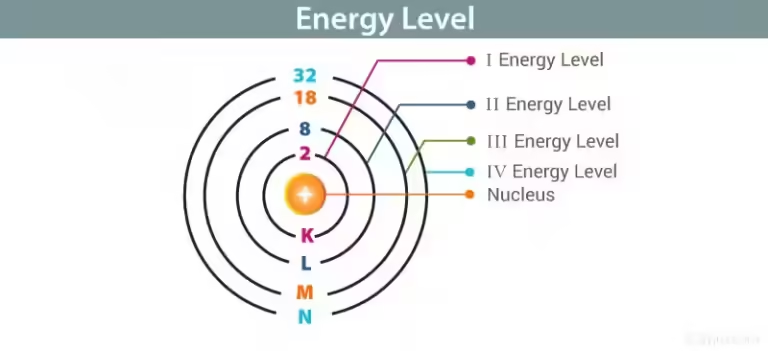
Imagine a bustling marketplace where merchants trade goods. In a chemical reaction, atoms are like merchants exchanging electrons, the currency of chemical interactions. When atoms gain electrons, they become reduced, and their energy levels increase. This excitement can manifest as a visible color change.
A red reaction often arises from the presence of transition metals, such as iron or copper. These metals have unfilled d orbitals, which allow them to absorb specific wavelengths of light and reflect others. When a red reaction occurs, the molecules involved have absorbed blue light, leaving red light to be reflected back to our eyes.
Think of a rusty nail. The iron in the nail reacts with oxygen in the air, forming iron oxide, commonly known as rust. The process involves a redox reaction where iron loses electrons and oxygen gains them. The resulting iron oxide has a reddish-brown hue, a telltale sign of a red reaction.
What is a White Reaction?
A white reaction typically occurs when a precipitate is formed. A precipitate is an insoluble solid that forms when two solutions are mixed. This insoluble compound often appears white, reflecting all wavelengths of light equally.
Consider the reaction between lead nitrate and sodium chloride. When these solutions are mixed, a white precipitate, lead chloride, forms. The white color arises because the lead chloride crystals have a high refractive index, scattering all wavelengths of light equally.
The Color Spectrum: A World of Reactions
While red and white are common colors in chemical reactions, the world of chemistry is full of vibrant and unexpected hues. Blue reactions can be attributed to the presence of copper ions, while green reactions often involve nickel compounds.
The color of a reaction is a direct reflection of the energy levels of the molecules involved. By understanding the principles of electron transfer and the unique properties of different elements, we can unlock the secrets behind the dazzling colors of the chemical world.
Exploring Further: Examples and Applications
The study of colorimetric reactions is crucial in various fields, from analytical chemistry to medical diagnostics.
- Titration: In acid-base titrations, the color change of an indicator solution signals the endpoint of the reaction.
- Blood Tests: Hemoglobin, the protein responsible for oxygen transport in blood, gives blood its characteristic red color. Changes in hemoglobin levels can be detected by analyzing the color of blood samples.
- Environmental Monitoring: Heavy metal pollution can be detected using colorimetric methods, allowing for swift identification and mitigation efforts.
The study of color reactions is a constant reminder that the world of chemistry is full of vibrant surprises, waiting to be discovered.
Frequently Asked Questions: White vs. Red Reaction
What is a white reaction?
A white reaction is a type of reaction that occurs when the reactants are mixed together and the resulting solution turns white. This is often due to the formation of a precipitate, which is a solid that forms out of solution.
What is a red reaction?
A red reaction is a type of reaction that occurs when the reactants are mixed together and the resulting solution turns red. This is often due to the formation of a colored complex ion, which is an ion that contains a metal atom surrounded by ligands.
What is the difference between a white and red reaction?
The main difference between a white and red reaction is the color of the resulting solution. White reactions typically result in a white precipitate, while red reactions typically result in a red solution. This difference in color is due to the different chemical species that are formed in each reaction.
What are some examples of white reactions?
Some examples of white reactions include the reaction of silver nitrate with sodium chloride to form silver chloride (a white precipitate), and the reaction of barium chloride with sulfuric acid to form barium sulfate (a white precipitate).
What are some examples of red reactions?
Some examples of red reactions include the reaction of iron(III) chloride with potassium thiocyanate to form a red complex ion, and the reaction of copper(II) sulfate with ammonia to form a red complex ion.