The Art of Predicting Chemical Reactions: Unraveling the Mystery of Major Products

Imagine a world where you could predict the outcome of a chemical reaction with absolute certainty. No more guesswork, no more trial and error. This is the dream of chemists everywhere, and while achieving perfect prediction might remain elusive, the field of organic chemistry has developed powerful tools to guide us toward the most likely outcome: the major product.
To understand the concept of a major product, think of a chemical reaction as a complex dance between molecules. These molecules collide, interact, and sometimes rearrange their atoms to form new molecules. Just like a dance, there might be multiple possible outcomes, but some moves are more likely than others. The major product represents the most probable outcome, the most likely "dance move" that will occur. Let's delve deeper into the factors that influence this intricate dance and reveal the secrets of predicting the major product.
The Guiding Principles: Understanding Reaction Mechanisms
At the heart of predicting the major product lies the understanding of reaction mechanisms. These mechanisms are like detailed choreography for the molecular dance, outlining the step-by-step process of bond breaking and forming. By dissecting the mechanism, we gain insight into the factors that favor the formation of specific products.
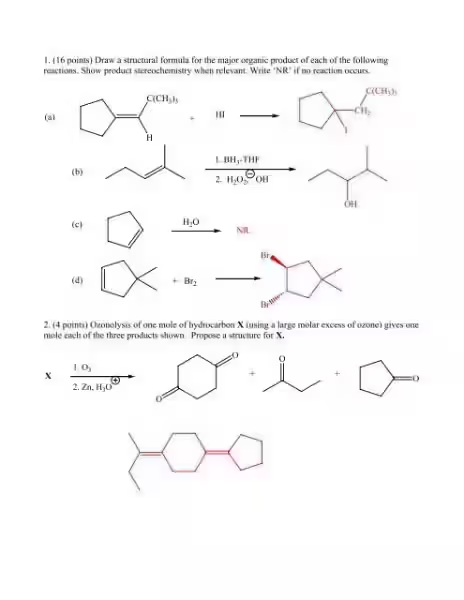
Consider a simple example: the addition of a hydrogen halide (like HCl) to an alkene. We know that the hydrogen atom from the acid will attach to one carbon of the double bond, while the halide will attach to the other. But which carbon gets the hydrogen? This is where the Markovnikov's Rule comes into play.
Markovnikov's Rule: A Guiding Star for Major Product Prediction
Markovnikov's Rule, a fundamental principle in organic chemistry, states that in the addition of a protic acid to an unsymmetrical alkene, the hydrogen atom will attach to the carbon atom with the most hydrogen atoms already attached.
Let's illustrate this with an example. Consider the reaction of propene (CH3CH=CH2) with hydrogen bromide (HBr). According to Markovnikov's Rule, the hydrogen atom from HBr will attach to the terminal carbon (the end carbon), which already has two hydrogen atoms. The bromine atom will then attach to the middle carbon, resulting in the formation of 2-bromopropane as the major product.
Why does this happen? The mechanism reveals the answer. The addition of the hydrogen halide proceeds through a carbocation intermediate. This carbocation is a positively charged carbon atom, and the more substituted the carbocation (having more alkyl groups attached to it), the more stable it is. The stability of the carbocation dictates the direction of protonation, leading to the formation of the most stable intermediate and thus the major product.
Beyond Markovnikov’s Rule: Unmasking Other Factors
While Markovnikov's Rule is a powerful tool, it's not the only factor influencing the major product. Several other aspects play a crucial role, and understanding them broadens our ability to predict reaction outcomes.
One such factor is steric hindrance. Steric hindrance refers to the space occupied by atoms and groups attached to a molecule. If a reaction pathway involves the formation of a crowded intermediate, it will be less favorable, leading to the formation of a less hindered product as the major product.
Another essential factor is stereochemistry. Stereochemistry deals with the three-dimensional arrangement of atoms in molecules. Reactions can produce different stereoisomers (molecules with the same connectivity but different spatial arrangement), and understanding the reaction mechanism helps predict which stereoisomer will be the major product.
Navigating the Labyrinth of Reactions: A Framework for Predicting Major Products
The prediction of major products is a vital skill for organic chemists. By understanding reaction mechanisms, applying principles like Markovnikov's Rule, and considering other factors like steric hindrance and stereochemistry, we can navigate the labyrinth of chemical reactions with greater confidence.
Remember, predicting the major product is not just about memorizing rules. It's about developing a deep understanding of the fundamental principles that govern chemical reactions. With this understanding, you can become a master of predicting reaction outcomes and unlock the world of organic chemistry.