The Sun's Energy: The Driving Force Behind Earth's Heat
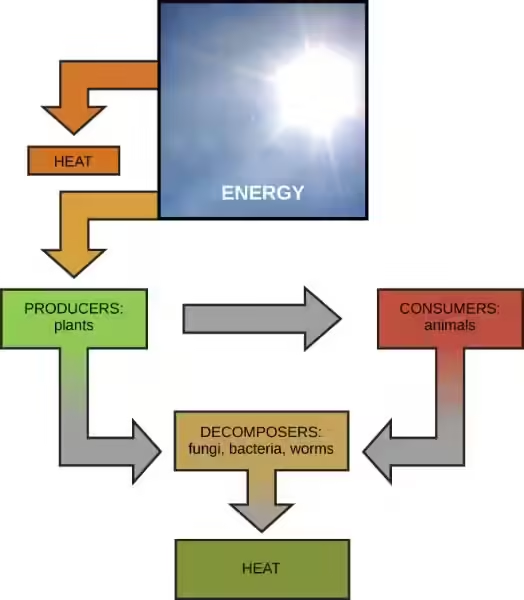
Our planet Earth is a vibrant and dynamic place, teeming with life. At the heart of this activity lies the Sun, a colossal ball of burning gas that provides us with the energy we need to survive. The sun's energy, which travels to Earth as light and heat, is responsible for everything from the weather patterns we experience to the growth of plants that sustain our food chain.
But how does this energy from the sun transform into heat on Earth? It's a fascinating process that involves a combination of physical mechanisms and atmospheric interactions.
The Sun’s Radiation: The Source of Earth’s Heat
The sun's energy, which we experience as light and heat, is released through a process called nuclear fusion. In the sun's core, hydrogen atoms collide and fuse together to form helium, releasing an immense amount of energy in the process. This energy travels through space as electromagnetic radiation, a form of energy that can travel through a vacuum.
When this radiation reaches Earth, a portion of it is absorbed by the Earth's atmosphere and surface. This absorbed energy warms the planet, driving the climate and weather systems we experience. However, not all of the sun's radiation reaches the Earth's surface.
The Earth's Atmosphere: A Protective Shield
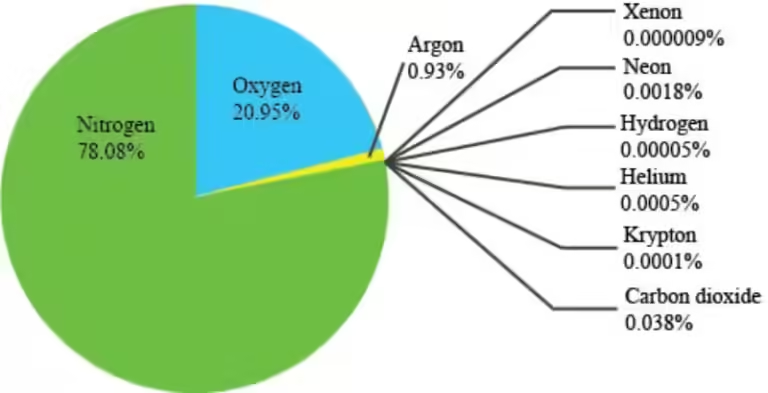
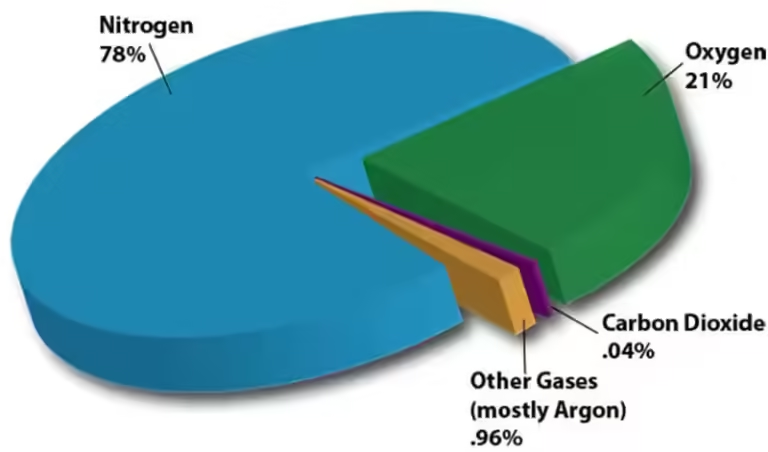
Our atmosphere acts as a shield, filtering and absorbing some of the incoming solar radiation. Different gases in the atmosphere have different abilities to absorb and reflect radiation. For instance, ozone gas in the stratosphere absorbs harmful ultraviolet radiation, protecting us from its damaging effects. Other gases, like carbon dioxide and methane, absorb infrared radiation, contributing to the greenhouse effect that keeps our planet warm enough to sustain life.
The Earth's atmosphere is composed of several layers, each with its unique characteristics. The troposphere, the lowest layer, is where most of the weather occurs. This layer is heated from below by the Earth's surface, creating convection currents that drive our weather patterns. Above the troposphere is the stratosphere, where the ozone layer absorbs ultraviolet radiation. The temperature in the stratosphere increases with altitude due to the absorption of this radiation.
Mechanisms of Heat Transfer: From Sunlight to Earth’s Temperature
Once the sun's energy reaches Earth, it is distributed and transformed through various processes, including:
Conduction: Heat Transfer Through Direct Contact
Imagine placing a metal spoon in a hot cup of tea. The heat from the tea is transferred to the spoon through conduction, where the molecules of the hot tea vibrate and cause the molecules of the spoon to vibrate as well, increasing its temperature. This same principle applies to the Earth's surface, where heat from the sun is conducted into the ground and rocks.
Convection: Heat Transfer Through Fluid Movement
Convection is another important mechanism of heat transfer, especially in fluids like air and water. Think of a pot of boiling water. As the water at the bottom of the pot is heated, it becomes less dense and rises, while the cooler water at the top sinks to take its place. This creates a circular motion, transferring heat throughout the water. Similarly, the Earth's surface is heated by the sun, causing air to warm and rise, creating convection currents that drive our weather patterns.
Radiation: Heat Transfer Through Electromagnetic Waves
Radiation is the transfer of heat through electromagnetic waves. The sun's energy travels to Earth as radiation, and the Earth's surface also emits radiation in the form of infrared waves. This is the same radiation that we feel as heat. The amount of radiation emitted by a surface depends on its temperature. Warmer objects emit more radiation than cooler objects.
These three mechanisms of heat transfer work together to distribute the sun's energy throughout the Earth's atmosphere and surface, creating the complex climate and weather patterns we experience.
The Impact of Heat Transfer: Climate and Weather Patterns
The uneven distribution of the sun's energy across the Earth's surface drives the global climate and weather patterns. The tropics, which receive more direct sunlight, are generally warmer than the poles, which receive less direct sunlight. This temperature difference creates pressure gradients in the atmosphere, driving the winds and ocean currents that distribute heat around the globe.
The heat transfer processes discussed above play a critical role in shaping our climate. For example, the greenhouse effect, caused by the absorption of infrared radiation by gases like carbon dioxide, contributes to the warming of the Earth's atmosphere. This warming can lead to changes in weather patterns, such as more frequent and intense heat waves, droughts, and storms.
Understanding how the sun's energy is transformed into heat on Earth is crucial for comprehending the complexities of our planet's climate and the challenges we face in addressing climate change. By studying these processes, we can better understand the impacts of human activities on our environment and work towards sustainable solutions.
The Power of Light: Exploring Heat Absorption and Reflection
Have you ever wondered why some surfaces feel hot under the sun while others stay cool? It's all about how light interacts with different materials. This activity helps us understand the fascinating relationship between light and heat.
Imagine sunlight as a stream of tiny energy packets called photons. When these photons hit a surface, they can be either absorbed or reflected.
Absorption: When a material absorbs light, it means the photons transfer their energy to the material's atoms. This causes the atoms to vibrate faster, generating heat. Think of a dark asphalt road on a sunny day - it gets incredibly hot because it absorbs a lot of sunlight.
Reflection: Conversely, when light is reflected, the photons bounce back without transferring their energy to the material. This is why a white shirt feels much cooler than a black shirt in the sun. White surfaces reflect a majority of the light, while darker surfaces absorb more.
This experiment allows us to observe these phenomena firsthand. By exposing different materials to sunlight, we can measure their temperature changes. Materials that absorb more light will experience a greater temperature increase, while materials that reflect more light will remain relatively cool.
Here are some key takeaways from this experiment:
- Color Matters: Darker colors tend to absorb more light and heat up faster than lighter colors, which reflect more light. This explains why black clothing is often associated with warmth and white clothing with coolness.
- Material Matters: The composition of a material also plays a significant role in its ability to absorb or reflect heat. For example, metal surfaces are generally good heat conductors and will warm up quickly when exposed to sunlight, while materials like wood or plastic are better insulators and will heat up more slowly.
- Practical Applications: Understanding how light interacts with materials has numerous practical applications. Choosing light-colored clothing in summer, using reflective materials for building insulation, and designing solar panels to maximize light absorption are all examples of how this knowledge can be utilized.
By conducting this simple experiment, we gain a deeper understanding of the relationship between light and heat. We learn that not all materials react to sunlight in the same way, and this difference in absorption and reflection has real-world implications.
How does the sun's energy transform into heat?
The sun's energy, in the form of light, is absorbed by materials. The absorbed light energy causes the atoms within the material to vibrate faster, which generates heat.
Why does a black shirt feel hotter than a white shirt in the sun?
Darker colors, like black, absorb more light energy, leading to increased atomic vibration and heat generation. White surfaces, on the other hand, reflect more light, minimizing heat absorption.
What are some practical applications of understanding light absorption and reflection?
This knowledge can be used to design solar panels for efficient energy capture, choose light-colored clothing for staying cool in the summer, and utilize reflective materials for building insulation.