What's the Difference Between Gas and Vapor? Unveiling the Subtle Distinctions
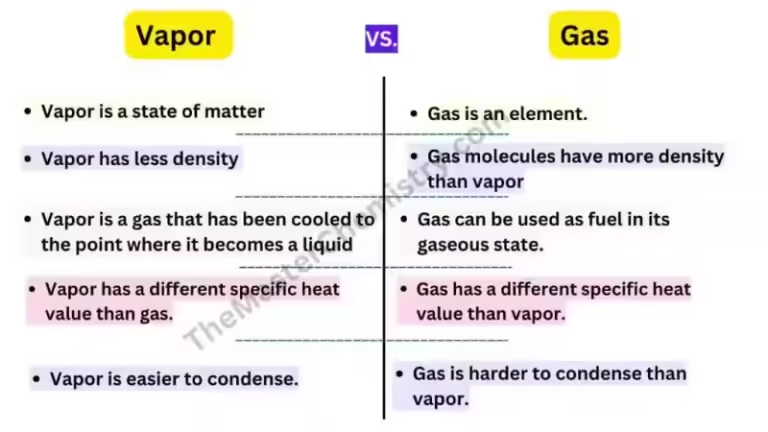
In the realm of chemistry and physics, the terms "gas" and "vapor" are often thrown around interchangeably, leading to confusion. While both describe a state of matter where molecules are in constant motion and spread out, there's a subtle but crucial distinction that sets them apart.
This distinction hinges on the substance's natural state at room temperature. It's like the difference between a puddle of water and a cloud: both consist of water molecules, but the cloud is water in its vapor form, while the puddle is water in its liquid form.
Vapor: The Gaseous Form of Liquids and Solids
Imagine a steaming cup of hot coffee. The "steam" rising from the coffee is actually water vapor. Water, which is typically a liquid at room temperature, has been heated to its boiling point and transitioned into its gaseous state. This gaseous form is what we call vapor.
Similarly, imagine dry ice, which is solid carbon dioxide. When dry ice sublimates (directly transitioning from a solid to a gas), it becomes carbon dioxide vapor. This illustrates that vapor can also be the gaseous form of a solid.
Key Features of Vapor:
- Origin: Vapors are formed by heating a liquid or solid to its boiling point or sublimation point, respectively.
- Condensation: Vapors can condense back into their liquid or solid form when the temperature drops or pressure increases. Think of how steam turns back into water droplets when it touches a cold surface.
- Room Temperature State: The substance that forms the vapor is normally a liquid or solid at room temperature.
Gas: A Naturally Gaseous State
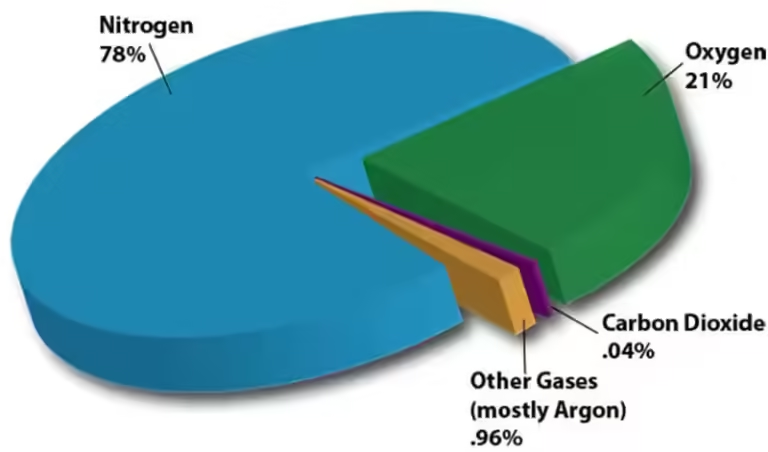
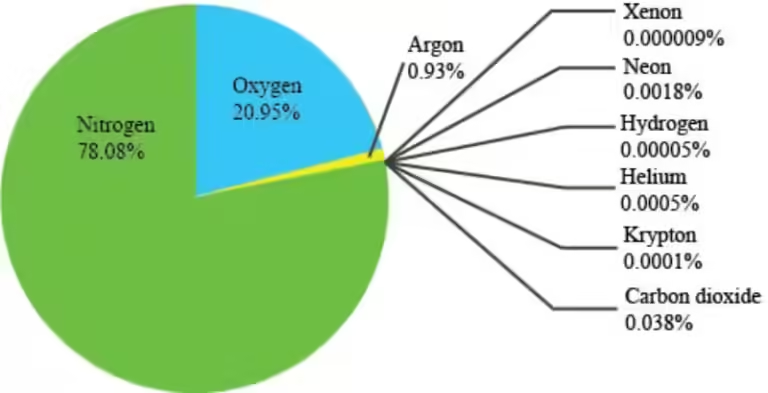
Unlike vapor, a gas exists in its gaseous state naturally at room temperature. Examples include oxygen, nitrogen, and helium. These substances remain in their gaseous form even at room temperature and pressure.
Think of the air we breathe. It's primarily composed of nitrogen and oxygen, both of which are gases at room temperature. They don't need to be heated to achieve their gaseous state; they naturally exist as gases.
Key Features of Gas:
- Room Temperature State: The substance exists as a gas at room temperature.
- Condensation: Gases do not readily condense back into their liquid or solid form under normal conditions. They require extreme pressure or low temperatures to liquefy.
- Critical Temperature: Gases exist above their critical temperature, a point where they cannot be liquefied regardless of pressure.
The Importance of Understanding the Difference
The distinction between vapor and gas is more than just a semantic quirk. It's crucial for understanding the behavior and properties of different substances under various conditions.
For instance, the term "vapor" is often used in everyday language to describe the gaseous form of substances that are typically liquid at room temperature, while "gas" is used more in scientific contexts. Understanding this distinction can help you interpret scientific literature and make informed decisions regarding the handling and storage of different substances.
Illustrative Examples:
- Water: At room temperature, water is a liquid. When heated to its boiling point, it turns into water vapor (steam). When the steam cools down, it condenses back into liquid water.
- Mercury: Mercury is a liquid at room temperature. When heated, it becomes mercury vapor. This vapor is used in mercury vapor lamps, which create light by passing electricity through the vapor.
- Dry Ice: Dry ice is solid carbon dioxide. When it sublimates (directly transitioning from a solid to a gas), it becomes carbon dioxide vapor. This vapor is used for various purposes, such as creating fog effects and preserving food.
Final Thoughts
Understanding the difference between vapor and gas is essential for comprehending the behavior of substances in different states of matter. Remember, vapor is the gaseous form of a substance that is normally a liquid or solid at room temperature, while gas is a substance that naturally exists in its gaseous form at room temperature.
Next time you encounter these terms, take a moment to consider the context and the substance in question. This will help you grasp the subtle but significant distinction between vapor and gas, enhancing your understanding of the world around you.
Frequently Asked Questions about Vapor and Gas
What is the difference between vapor and gas?
While both vapor and gas are states of matter where molecules move freely, vapor is the gaseous form of a substance that is normally a liquid or solid at room temperature. This means vapor can condense back into its liquid or solid form if the temperature is lowered or the pressure is increased. Gases, on the other hand, are substances that exist naturally in a gaseous state at room temperature.
Why is it important to differentiate between vapor and gas?
Understanding the distinction between vapor and gas is crucial for comprehending the behavior and properties of various substances under different conditions. This knowledge is particularly important in fields like chemistry, physics, and engineering.
What is the critical point in relation to vapor and gas?
A substance is considered a gas when it is above its critical temperature, meaning it cannot be liquefied by pressure alone. A substance is considered a vapor when it is below its critical temperature.
Can vapor be liquefied?
Yes. Vapors can be liquefied by increasing pressure or decreasing temperature. This is because vapors exist below their critical temperature, allowing them to condense back into their liquid or solid form.
Can gas be liquefied?
Gases above their critical temperature cannot be liquefied by pressure alone. However, they can be liquefied by decreasing the temperature to a point where they are below their critical temperature.
What are some examples of vapor?
Common examples of vapor include water vapor (steam), iodine vapor, and mercury vapor.
What are some examples of gas?
Common examples of gases include oxygen, nitrogen, helium, and carbon dioxide.
Is steam a vapor or a gas?
Steam is considered a vapor because it is the gaseous form of water, which is a liquid at room temperature.
Is dry ice a vapor or a gas?
Dry ice (solid carbon dioxide) sublimates directly into carbon dioxide gas. While carbon dioxide is gaseous at room temperature, dry ice is a solid, making the transition a sublimation process, not vaporization.